THE CHALLENGE
Running clinical studies has traditionally been time-consuming and inefficient for both the research study coordinator and study participant. Recruiting, consenting, enrolling, and monitoring participants’ activities during a clinical study requires the coordinator to use a mix of stand-alone software tools to keep track of patients’ onboarding and study engagement. They spend hours daily checking participants’ activity, manually logging into device accounts and individually notifying those who forgot to wear or sync their wearable devices or complete their study tasks. For participants, study information, notifications, and instructions can be overwhelming and discourages continued participation.
THE OUTCOME
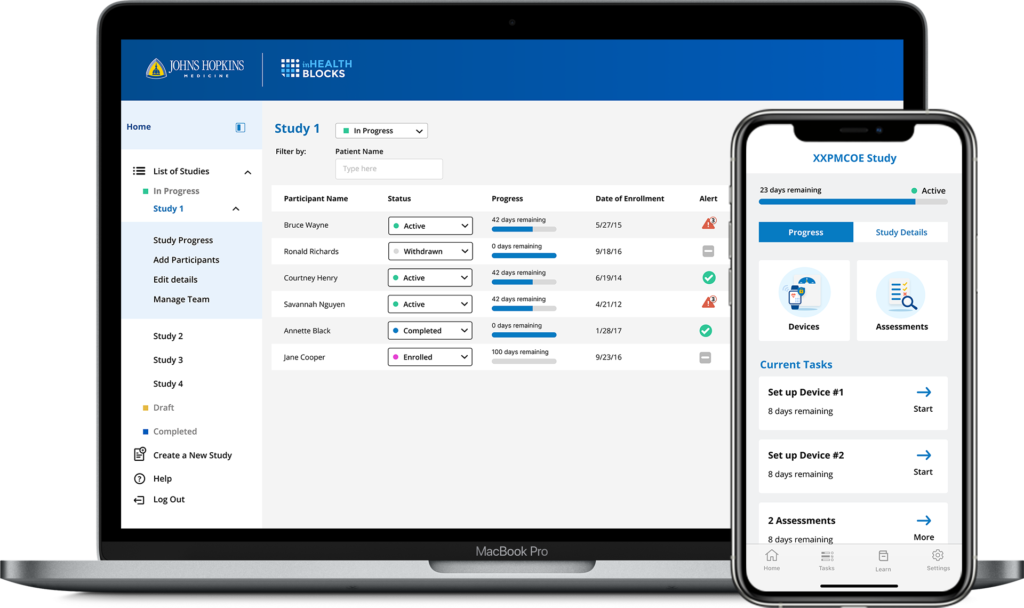
inHealthBlocks integrates remote patient monitoring, medical wearables, and study management in one place. The platform includes a dashboard to streamline research operations for the study coordinators, who can create studies, add participants, and set up devices while also creating and managing notifications and reminders. Study participants use the native mobile application to stay engaged over the course of the research study. They can review study details and instructions, and configure their preferred communication channel (SMS or email) for reminders and encouragement.